Acasti Pharma (ACST) to report phase results, is a biopharmaceutical innovator focused on the research, development and commercialization of prescription drugs using omega-3 fatty acids derived from krill oil. Omega-3 fatty acids have extensive clinical evidence of safety and efficacy in lowering triglycerides in patients with hypertriglyceridemia. Acasti is focused on addressing this important unmet market need.
Acasti Pharma (ACST) Phase 3 Update (Release December 2019)
Acasti Pharma has entered into phase 3 of it’s trials for it’s lead candidate CaPre (omega-3 phosholipid) which the company is developing for treatment of severe hypertriglyceridemia, a condition characterized by abnormally high levels of triglycerides in the bloodstream (over 500 mg/dL).
The analysis gathered from the trials are due to report top-line results for TRILOGY 1 in December 2019 and TRILOGY 2 in January 2020, if results for TRILOGY 1 aren’t by the end of December the schedule may be pushed up until the end of January 2020.
Topline results will include a readout of the primary endpoint, which is intended to show CaPre’s overall impact on lowering triglycerides (TGs) after 12 weeks compared to placebo. Safety and tolerability (e.g. overall adverse events (AE) and serious AE rate, any discontinuation due to AEs, and AEs of special interest such as gastrointestinal events) will also be reported.
Further catalysts aside from TRILOGY 2 are the release of secondary and exploratory endpoints in March 2020, presentation of the full dataset at “important” scientific meetings and, most importantly, the filing of a new drug application to obtain FDA approval for release into the U.S. market. The Company has stated that it intends to work with partners overseas with regards to the licensing/distribution of CaPre with Asia in particular being seen as a key market with funding commitments already negotiated in the vast Chinese market where ACST is already patent protected (other patents have been obtained in Mexico, Israel and other many other countries pending).
Acasti Pharma (ACST) Phase 2 Results
In two separate Phase 2 clinical trials conducted by Acasti in Canada (the COLT and TRIFECTA trials), CaPre was found to be safe and well-tolerated at all doses tested, with no serious adverse events that were considered treatment-related. Among the reported adverse events with an occurrence of greater than 2% of subjects and greater than placebo, only diarrhea had an incidence of 2.3%.
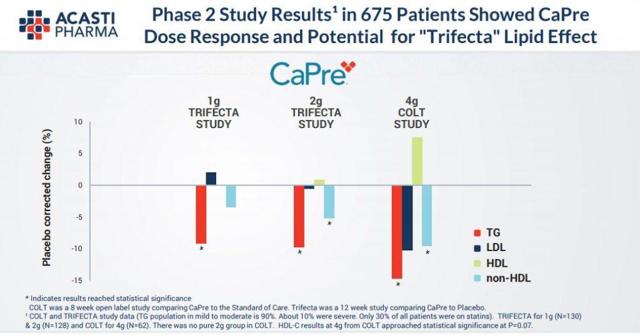
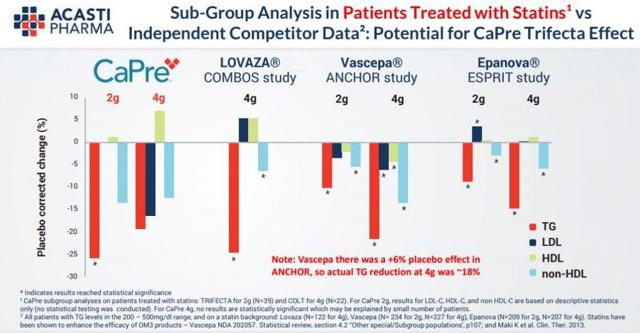
In both Phase 2 clinical trials, CaPre significantly lowered TGs in patients with mild to severe HTG. Importantly, in these studies, CaPre also demonstrated potentially beneficial effects on LDL-C, non-HDL-C, and HDL-C.
Acasti Pharma Final Thoughts
Our analysts claim that ACST is breaking out of a long basing pattern and embarking on a strong uptrend over the past few weeks evidenced by higher highs, higher lows, and rising volume. Our analysts also believe the word is out that the trial went well, and smart money continues to buy on “anticipation” of the “news”. While fear of missing out (FOMO) sets in, this situation is different than other P3 data releases, since ACST is performing not one, but two, trials, with the second one and complete data releases in 1Q20 to provide the one-two punch that could send ACST to further high trading ranges after this first P3 trial outcome is released near-term.
From an immediate target viewpoint, the breaking of $2.25-$2.50 and strong recent uptrend off the lows of the year suggest a move to $5+ quickly. There is a 2014 gap around $9-$10 and which would match our valuation analysis of $1BN supported by T1/T2 outcomes.
The Company has an estimated $25MM cash, expected $15MM from warrant exercises, and potential prepayments/license payments from foreign BP players looking to launch CaPre in their own markets which would be non-dilutive financing. While we would not be surprised to see an equity offering in 2020, it is not required for ACST to move through T1/T2 datasets and applications to the FDA for launch of CaPre to high risk patients in 2020. In the meantime, it appears that smart money agrees and has been piling into the stock with insider/institutional ownership % rising from 14% to 30%+ as per recent filings.
Recommended: Should you buy Outlook Therapeutics in 2020?